近日,2023年第31届国际血栓与止血学会(ISTH)年会在加拿大魁北克蒙特利尔举行。作为首位在ISTH会议上就中国自主研发产品做大会演讲的中国临床PI,中国医学科学院血液病医院杨仁池教授以口头报告形式公布一项有关晟斯生物的超长效重组凝血因子产品注射用培重组人凝血因子VIII-Fc融合蛋白的临床研究结果,为全球一百多万患者带来更多更好的选择,让患者不仅能解除病痛,更能回归正常的生活。

满足临床需求,提升患者用药依从性
血友病是一组凝血因子缺乏导致凝血功能障碍的遗传性出血性疾病,也是严重危害健康的出生缺陷疾病。患者临床常表现为自发性出血或轻度外伤后出血不止,极易导致患者残疾,严重者可危及生命,血友病患者也因此被称为“玻璃人”。全球血友病患者超过一百万人,其中中国血友病患者约有14万人,这些患者都需要终身注射凝血因子。
第一代血友病治疗药物为血源性凝血因子制剂,主要来源为血浆提取,存在血源感染、供给不足等问题。上世纪九十年代问世的第二代重组凝血因子制剂可有效降低血源性病毒传播的风险,提升产品的安全性。随着第三代长效凝血因子的出现,将进一步提升患者用药依从性。
注射用培重组人凝血因子VIII-Fc融合蛋白(代号:FRSW117)同时采用PEG修饰和Fc融合蛋白两种长效技术,成功实现“一周一次”预防治疗,是世界第二款、国产第一款在研的超长效重组八因子产品,让血友病A患者回归正常生活。产品具有自主知识产权,核心专利已经在多个国家和地区授权或进入实质审查阶段。
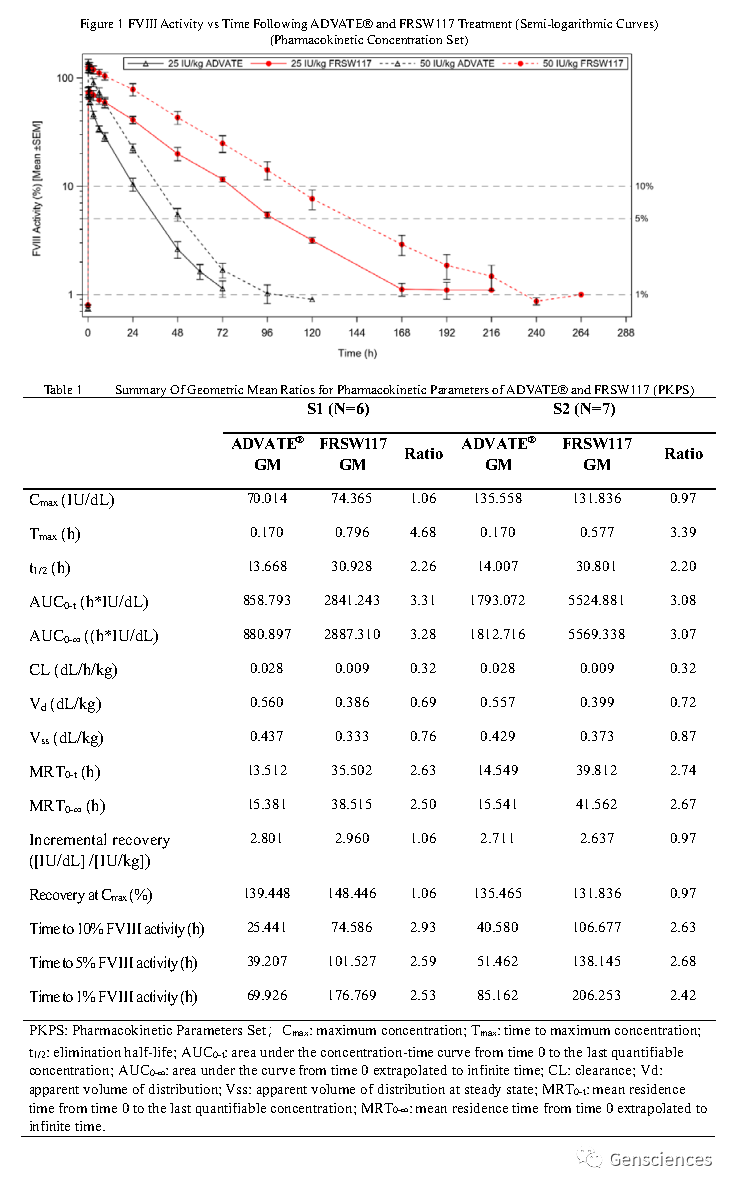
FRSW117可以实现一周一次的治疗效果,极大改善了患者的生活状况;在所有受试者中,未检测到八因子抑制物的存在。临床Ⅰ期研究数据显示,不同剂量的两组受试者(25 IU/kg和50 IU/kg)体内维持在1%八因子活性以上的时间与现有的商业化产品(ADVATE®)相比,分别延长了2.59倍和2.49倍。同时,两组受试者体内八因子的半衰期分别为ADVATE®产品的2.129倍和2.232倍。
Abstract原文如下:
A Phase 1 Study to Evaluate the PK, Safety and Tolerability of FRSW117 With Extended Half-Life in Patients With Severe Hemophilia A
NCT number: NCT04864743
Abstract ID: 1443652
OC 24 - Hemophilia Clinical – Factor Levels and Pharmacokinetics
Sunday, June 25, 2023,14:45 – 16:00, Eastern Time
Background: Factor VIII (FVIII) replacement therapy of severe hemophilia A (HA) necessitates multiple doses of FVIII products weekly to maintain a > 1% FVIII trough activity due to their short half-life. FRSW117 is a PEGylated FVIII Fc fusion protein engineered to extend its half-life so as to maintain a sufficient FVIII level and improve treatment outcomes.
Aims: To evaluate the pharmacokinetics, safety/tolerability, and immunogenicity of FRSW117 in patients with severe HA.
Methods: In this first-in-human, open label, single dose, self-controlled study, 13 previously treated males aged 12 (inclusive) to 65 years with severe HA (FVIII < 1%) were enrolled to receive ADVATE® followed by FRSW117, both at 25 IU/kg, 4 days apart (N=6, Group S1); or both drugs at 50 IU/kg, 6 days apart (N=7, Group S2), followed by a 4-week follow-up after FRSW117 injection. All patients provided written informed consents. The protocol was approved by each site’s IEC/IRB.
Results: The maximum concentration (Cmax) and area under the curve (AUC0-t) of FⅧ activity increased in a dose-dependent manner (Figure 1). The geometric mean elimination half-life of FRSW117 exceeded twice that of ADVATE® (30.928 hours vs. 13.668 hours in S1; 30.801 hours vs. 14.007 hours in S2) (Table 1). The mean FVIII level was > 5% for 101.527 hours in S1 and 138.145 hours in S2. Both groups maintained an FVIII activity of > 1% for longer than 168 hours (7 days), 176.769 hours for S1 and 206.253 hours for S2. No FⅧ inhibitors were detected and no hypersensitivity or anaphylaxis were reported. No serious adverse events or ≥ Grade 3 adverse events were reported. No spontaneous bleeding requiring intervention was reported within 9 days after FRSW117 treatment.
Conclusions: FRSW117’s pharmacokinetic characteristics supported a potential weekly treatment interval. It also displayed favorable safety/tolerability and immunogenicity profiles.